Synthesis StarterKit
for a successful use of diamond electrodes in the laboratory
Ideal for electrochemistry courses and internships at universities and schools
Due to its simple and modular concept, the Synthesis StarterKit is an ideal instrument for use in practical chemistry courses at universities and colleges, as well as for demonstration experiments at secondary schools. It enables students and pupils to already gain experience with high-tech and future-oriented electrode materials during their education. Furthermore, the practice-oriented cell concept illustrates the transition from concept to scalable electrosynthesis cell.
The Synthesis - StarterKit is a flexible and modular tool for flow electrolysis on a small laboratory scale. It is designed to support you at the beginning of the development of an electrochemical process and is robust, flexible and easy to use. Each part is available separately.
The system features a state-of-the-art DIACHEM® BDD anode to benefit from the unique properties of this electrode material. Due to its flexible design concept, you can expand the system to achieve the highest efficiency in the development of advanced electrochemical technology.
7 Application Notes for a Direct Start
Benefit from our experience: on the one hand seven test protocols teach basic working techniques and on the other hand allow you to perform first electrosyntheses in a simple way. This allows the user to become familiar with the system. The following experiments are available so far:
Advantages of our Synthesis StarterKit
modular design - each part can be purchased separately
equipped with DIACHEM® BDD anode (Boron Doped Diamond)
includes a laboratory power supply for the electrolysis cell as well as an adjustable power supply unit for fan and pump
Properties of our Synthesis StarterKit
| electrode | 26 mm diameter |
|---|---|
| size | 3.14 cm² active surface |
| seal | Viton® or Tealon® |
| weight | 0.8 kg |
| cylinder | 6 mm diameter |
| anode | DIACHEM® BDD-Anode |
| cathode | structured stainless steel for the electrolyte feed |
- Robust stainless steel for cathode end plates and anode contacting
- Fast opening and closing mechanism for tube fixing
- Interelectrode distance adjustable from 1 mm to 5 mm by seal thickness
- 3.14 cm² active electrode
- Only a few sealing parts for easy assembly and faultless operation
- Expandable for bipolar cell operation
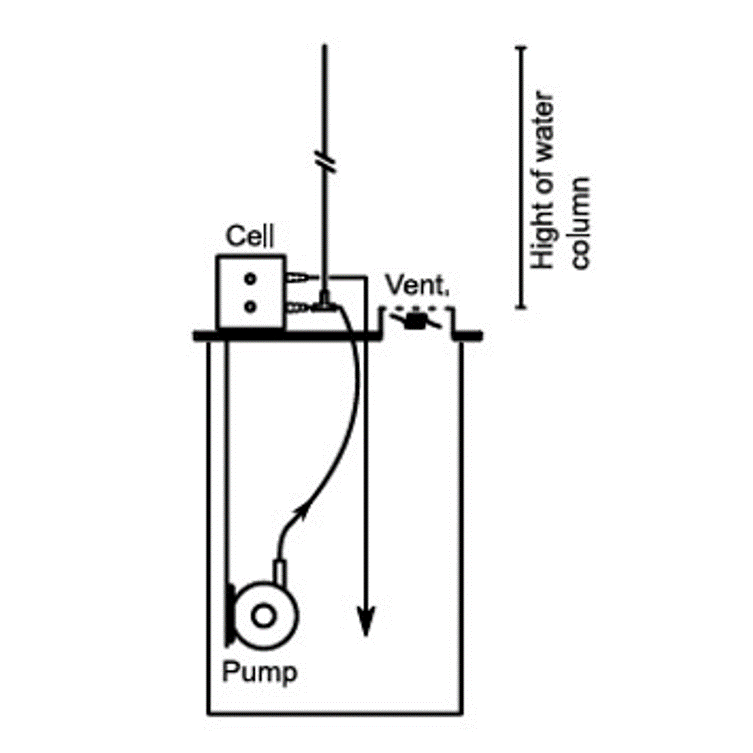
01 Recording Flow/Pressure Curve
Determining the pressure drop inside an electrochemical cell is an important factor.
This is mostly done in order to find suitable pump parameters and to prepare the cell scaleup and the related electrochemical process.
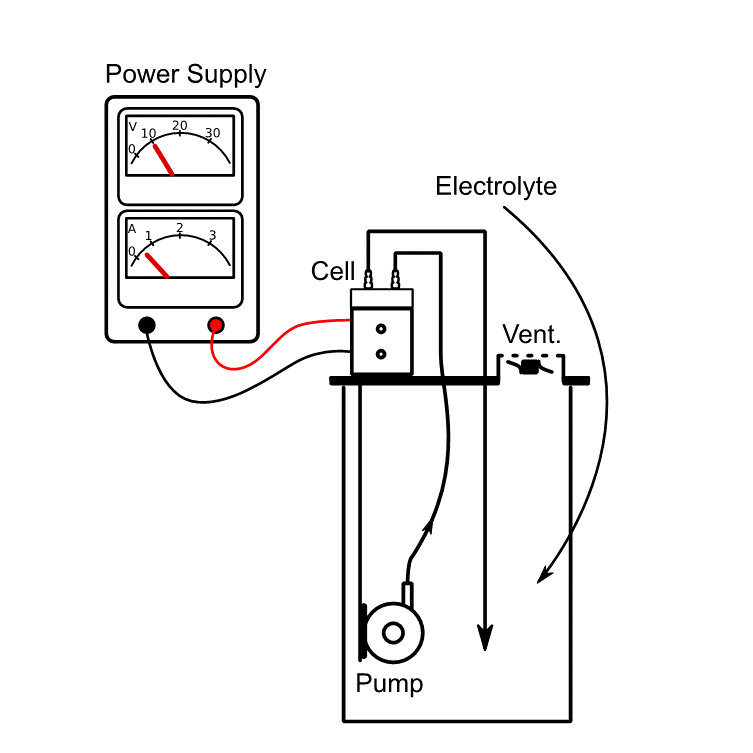
02 Recording Current/Voltage Curves
An electrochemical cell does not exhibit the electrical characteristics of a pure Ohmic system. The electrons pass several interfaces, mainly the electrode-electrolyte interfaces. Depending on the nature of the electrode reaction, the exponential behavior of the current/voltage curves can be significantly different at low current densities.
At higher current densities, the curve propagation begins to be linear and the slope of the curve describes the Ohmic voltage drop across the electrolyte.
03 Oxidative Transformation of Uranine
This Application Note describes the oxidative transformation of Uranine by using a BDD electrode. Oxidative reactions in organic chemistry are very common and some of them are highly linked to the electrode material.
The Syntheses StarterKit can be directly used for this experiment without any modifications.
04 Oxidation of Sodium Chlorate Solution
05 Production of Peroxodicarbonate
06 Production of Persulfate
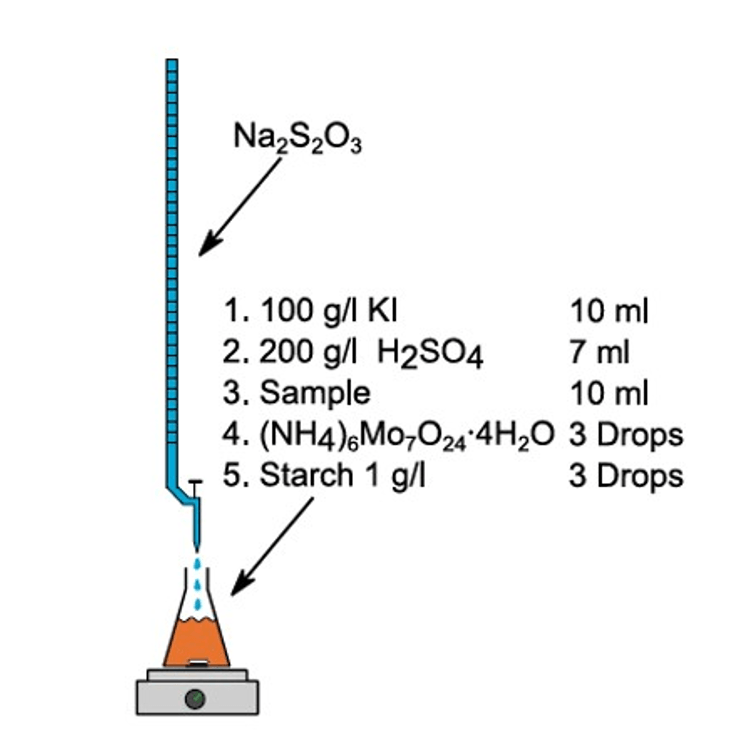
07 Titration of Oxidizers
This Application Note describes the iodometric titration of peroxodicarbonate (PODIC®) as an example for the determination of oxidants generated with the Synthesis StarterKit.
08 Using the Bipolar Extension Set (BES)
The Synthesis StarterKit’s monopolar cell is designed to be expanded to a bipolar cell using the Bipolar Extension Set (BES) which is basically an additional cathode and anode.
This offers an easy way to demonstrate the two concepts in real-life applications. All experiments described in other Application Notes can be performed with the bipolar Setup.
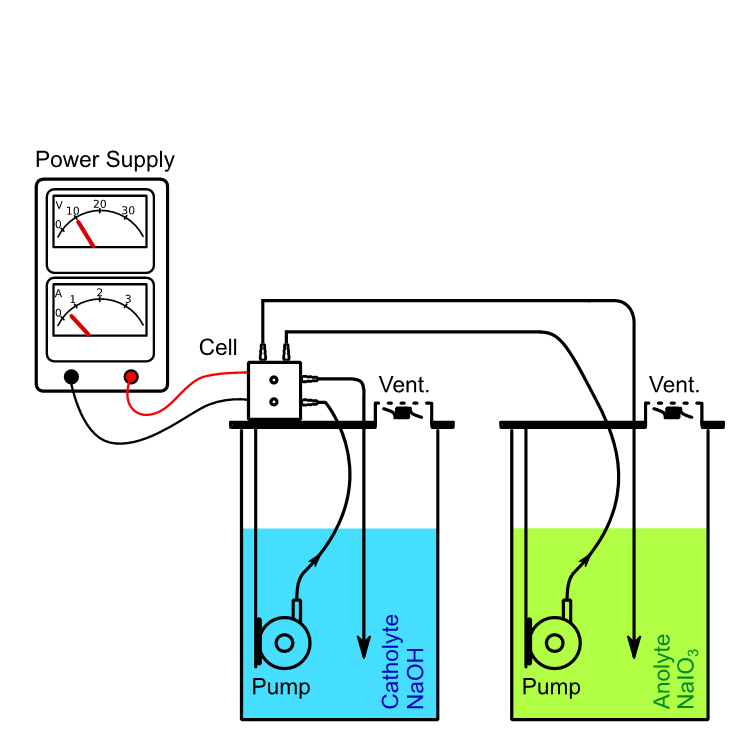
09 Using the Divided Cell Extension Set (DCES)
In a divided cell, anolyte and catholyte are separated by an ion conductive material. Examples are diaphragms or ion exchange membranes like Nafion®. The Divided Cell Extension Set (DCES) offers the possibility to extend the standard Synthesis StarterKit to a divided cell by providing a PTFE frame, a membrane and sealing material.
10 Production of PODIC® with the DCES
This application note describes the production of peroxo-dicarbonate (PODIC®) using a divided cell. For details regarding the divided cell, see App Note 09. The production of peroxo-dicarbonate (PODIC®) in an undivided cell as well as an overview of its chemical properties are described in App Note 05.
11 Using the Full Diamond Extension Set (FDES)
The Full Diamond Extension Set (FDES) offers the possibility to extend the standard Synthesis StarterKit to a cell with BDD anode and cathode by providing a PTFE frame, a sealing gasket, a conductive carbon felt and an additional BDD electrode.